PEP
A Practical Reference for Integrating HIV
and STI Testing Into Your Practice
This HIV Post Exposure Prophylaxis (PEP) section aims to provide clinicians with a comprehensive understanding of this critical intervention strategy. PEP refers to the administration of antiretroviral medications following potential exposure to the human immunodeficiency virus (HIV). Its primary goal is to prevent the establishment of HIV infection in a person who has experienced a non-occupational exposure that presents substantial risk for HIV transmission within the last 72 hours.
This section serves as a guide for healthcare providers, equipping them with the necessary knowledge to assess the eligibility of individuals for PEP, initiate timely treatment, and monitor patients throughout the course of therapy. By following evidence-based guidelines, healthcare professionals can play a pivotal role in reducing the risk of HIV transmission and improving patient outcomes.
What is nPEP?
nPEP (non-occupational post exposure prophylaxis) is the use of antiretroviral therapy following a known or potential exposure to HIV that occurred through sexual contact or through injection drug use.1
PEP Effectiveness
Studies, retrospective reviews, and expert opinion have suggested that daily adherence to a three-drug HIV PEP regimen of 28 days initiated within 72 hours of the potential HIV exposure is effective in preventing HIV infection.2,4,5
Cases of new HIV diagnoses despite use of PEP is attributed to delayed initiation beyond 72 hours or low daily adherence to regimen.2,4,5
PEP is for Emergency Situations
It’s important to note that PEP should not be considered a substitute for regular use of other HIV prevention methods.
PEP is not intended for individuals who may be frequently exposed to HIV.
If an individual is at ongoing risk for HIV due to repeated exposures, it is advisable to consult a healthcare provider about PrEP (pre-exposure prophylaxis).
Type of Exposures
Post-exposure prophylaxis should be recommended, and immediate medical attention should be sought, when an individual reports a known or potential exposure to HIV within the past 72 hours.1
Situations that warrant an immediate referral for PEP include:
Condomless receptive or insertive vaginal or anal intercourse with a partner living with HIV or a partner whose status was unknown, including intercourse that involved condom slippage or breakage and
Needle-sharing and
Injuries with exposure to blood or other potentially infected fluids from a source known to be HIV-infected or HIV status is unknown (including needlesticks with a hollow-bore needle, accidents, etc.)1
For persons presenting with wounds or needlestick injuries:
The site should be washed with soap and water, avoiding irritation of the skin.
The wound should not be “milked” or squeezed. Squeezing the wound may promote hyperemia and inflammation at the wound site, potentially increasing systemic exposure to HIV if present in the contaminating fluid.1
Lower risk exposures that require evaluation by a clinical provider on a case-by-case basis include:
Oral-vaginal contact (receptive and insertive)
Oral-anal contact (receptive and insertive)
Receptive penile-oral contact with or without ejaculation
Insertive penile-oral contact with or without ejaculation1
The level of risk in these situations increases with the presence of blood, genital ulcers, sexually transmitted diseases, or non-intact skin or mucus membranes.1
According to CDC PEP guidelines, individuals who report an ongoing risk of HIV exposure (e.g., through injection drug use or condomless sex) or who have used more than one course of PEP in the past year should be provided with resources for HIV prevention, including an assessment for PrEP eligibility.
For More Information
Updated Guidelines for Antiretroviral Postexposure Prophylaxis After Sexual, Injection-Drug Use, or Other Nonoccupational Exposure to HIV—United States, 2016
cdc.gov/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf- Clinical Guidance for PEP
cdc.gov/hivnexus/hcp/pep/?CDC_AAref_Val=https://www.cdc.gov/hiv/clinicians/prevention/prescribe-pep.html - PEPline: Expert consultation for issues and any other guidance on nonoccupational PEP can be obtained by calling the National Clinician Consultation Center’s Post-Exposure Prophylaxis PEPline at 888-448-4911
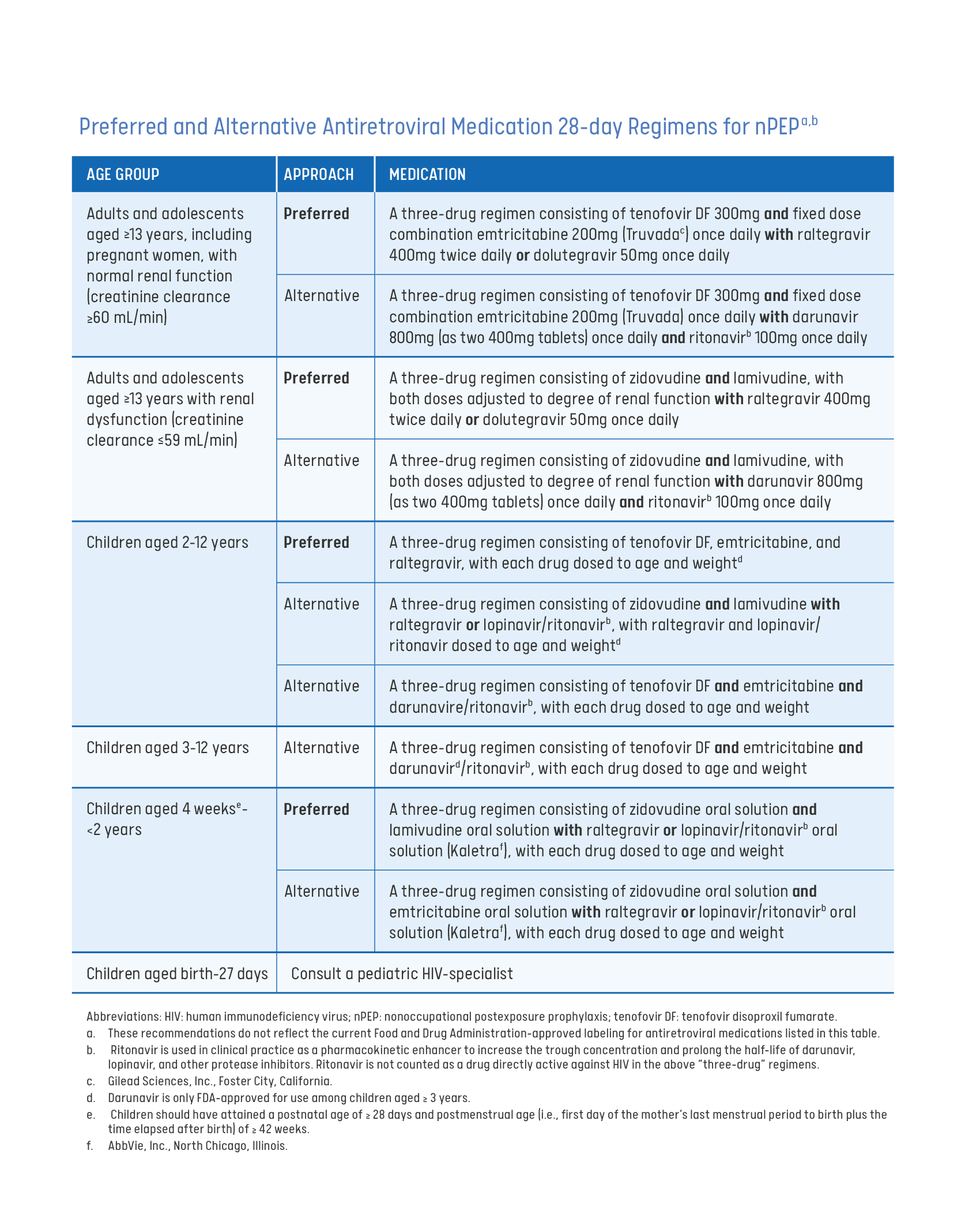
Prescribing PEP
This guide provides an evidence review, patient management guidelines, laboratory testing, recommended antiretroviral nPEP regimens, financial assistance for nPEP medication, and considerations for special populations.
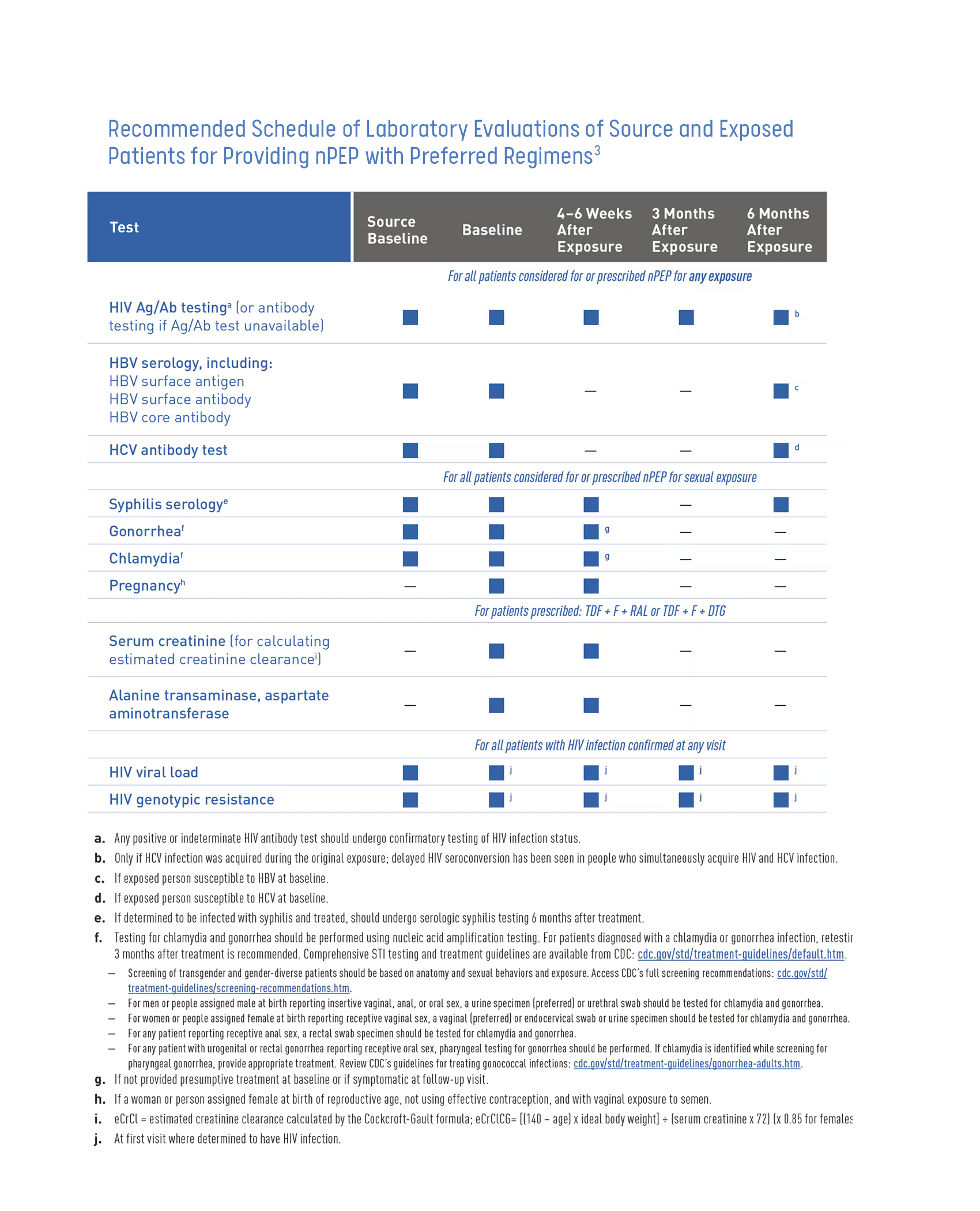
Baseline Labs and Monitoring
Baseline labs should be obtained at initial visit for PEP and monitored after completion of PEP regimen. See table below.3
Attempts should be made to also test the person believed to be the source of the possible exposure if possible.3
For More Information
- Philly’s 24/7 PEP hotline: 833-933-2815
Paying for PEP
Many insurance providers cover PEP.
If PEP is obtained through an emergency room, an individual may be given the first dose at the time of their visit, along with a few days’ supply. This will give them time to fill the prescription for the complete treatment course.
If an individual does not have insurance, their provider may be able to assist them with applying for medication assistance programs for PEP.
Enrollment applications that will be useful in applying for medication assistance programs are:
Gilead’s Advancing Access Form
services.gileadhiv.com/content/pdf/gilead_enrollment_form.pdfNASTAD’S Patient Assistance Tool
nastad.org/resources/pharmaceutical-company-patient-assistance-programs-and-cost-sharing-assistance-programsResources for Accessing nPEP
files.hiv.gov/s3fs-public/2023-10/PAP-CAP-Resources-for-Accessing-nPEP.pdf
Transition PEP to PrEP
Before completion of the patient’s 28-day PEP regimen, clinicians should assess patient’s interest in PrEP for ongoing HIV prevention.1
Negative HIV status needs to be confirmed prior to switching from PEP to PrEP, in addition to other baseline PrEP labs (see PrEP booklet). Otherwise, there is no contraindication to begin PrEP or need for a gap at the conclusion of the PEP regimen to start PrEP.1
Clinicians should discuss with their patient the importance of continuing adherence with PrEP regimen and changes to their medications when switching from PEP to PrEP.1
References
1. Centers for Disease Control and Prevention. (2016). Updated guidelines for antiretroviral postexposure prophylaxis after sexual, injection drug use, or other nonoccupational exposure to HIV— united states, 2016. In Centers for Disease Control and Prevention. U.S. Department of Health and Human Services.
cdc.gov/hiv/pdf/programresources/cdc-hiv-npep-guidelines.pdf
2. Jain, S., Oldenburg, C., Mimiaga, M., & Mayer, K. (2015). Subsequent HIV infection among men who have sex with men who used non-occupational post-exposure prophylaxis at a boston community health center: 1997–2013. AIDS Patient Care and STDs, 29(1), 20–25.
doi.org/10.1089/apc.2014.0154
3. Centers for Disease Control and Prevention. (2023). Algorithm for evaluation and treatment of possible nonoccupational HIV exposures. In Centers for Disease Control and Prevention.
cdc.gov/hiv/clinicians/prevention/prescribe-pep.html
4. Kahn, J., Martin, J., Roland, M., Bamberger, J., Chesney, M., Chambers, D., Franses, K., Coates, T., & Katz, M. (2001). Feasibility of postexposure prophylaxis (PEP) against human immunodeficiency virus infection after sexual or injection drug use exposure: The San Francisco PEP study. The Journal of Infectious Diseases, 183(5), 707–714.
doi.org/10.1086/318829
5. Roland, M. E., Neilands, T. B., Krone, M. R., Katz, M. H., Franses, K., Grant, R. M., Busch, M. P., Hecht, F. M., Shacklett, B. L., Kahn, J. O., Bamberger, J. D., Coates, T. J., Chesney, M. A., & Martin, J. N. (2005). Seroconversion following nonoccupational postexposure prophylaxis against HIV. Clinical Infectious Diseases, 41(10), 1507–1513.
doi.org/10.1086/497268