PrEP
An Overview of PrEP, Including its Efficacy, Indications, Monitoring Requirements, and Potential Side Effects
In order to keep up with the ever-changing landscape of HIV prevention, it is vital for clinicians to stay updated and equipped with the latest advancements in this field. One such advancement is the use of Pre-Exposure Prophylaxis (PrEP), which has become a critical tool in preventing the transmission of HIV. PrEP involves prescribing antiretroviral medications to reduce the risk of HIV transmission among anyone at risk for HIV infection. By following an oral or long-acting injectable regimen, PrEP has been proven to greatly decrease HIV acquisition.
This section aims to provide clinicians with a high-level overview of PrEP, including its efficacy, indications, monitoring requirements, and potential side effects. By incorporating PrEP into your clinical practice, you can make substantial strides towards reducing the incidence of HIV and improving the overall health outcomes of your patients and our community.
What is PrEP?
PrEP, or pre-exposure prophylaxis, is a pharmacological intervention that lowers the risk of acquiring HIV through sexual activity or injection drug use.
When taken as prescribed, PrEP proves to be highly effective in preventing HIV transmission.
Currently there are three FDA-approved PrEP medications available: two in oral form and one as a long-acting injectable:
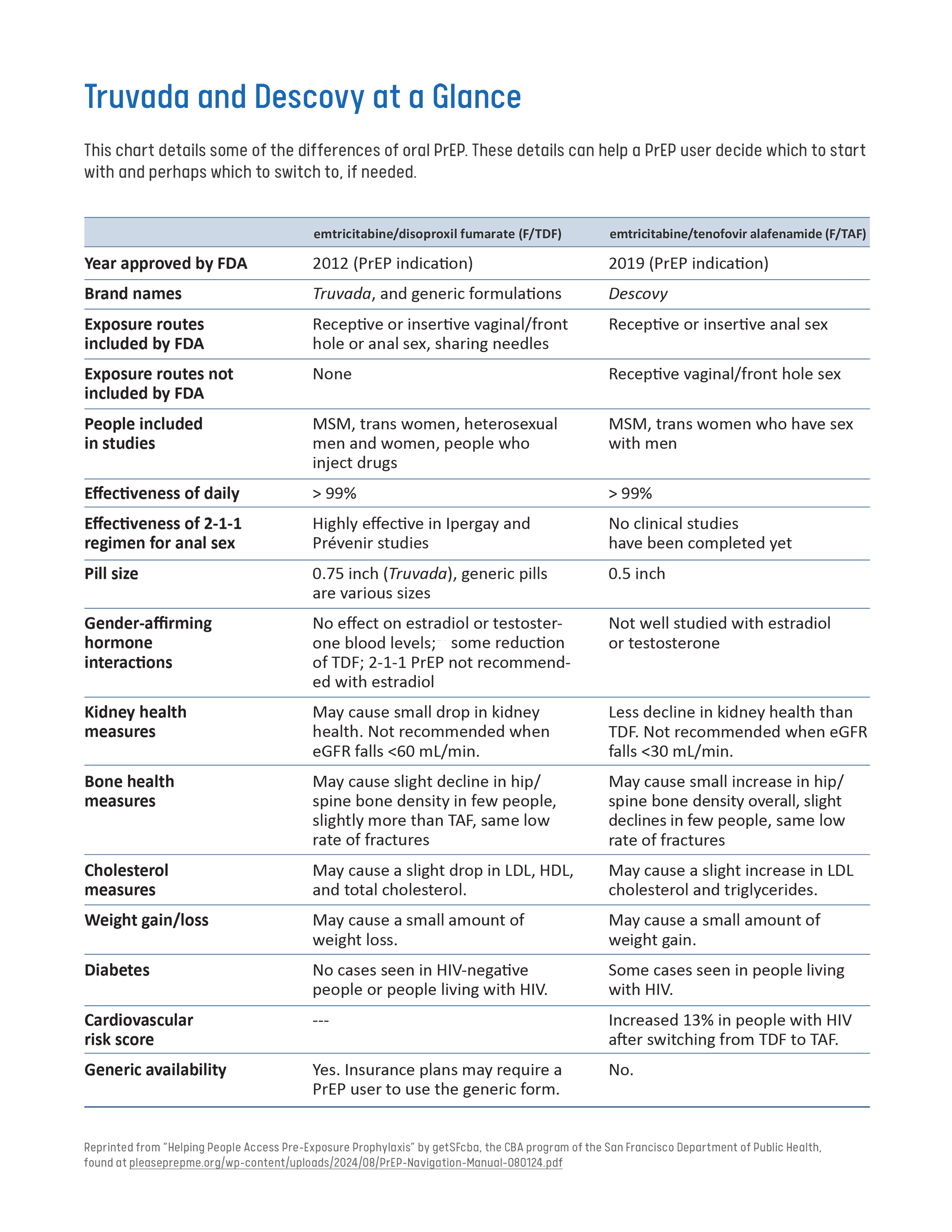
Truvada® (Tenofovir Dixoproxil Fumarate and Emtricitabine – TDF/FTC): This daily PrEP option is recommended for all adults and adolescents weighing at least 77 pounds. Taking one pill at the same time every day can significantly reduce the risk of HIV transmission through both sexual contact and injection drug use.
Descovy® (Tenofovir Alafenamide and Emtricitabine – TAF/FTC): Another one pill daily oral PrEP option recommended to substantially lower the risk of HIV acquisition through sexual activity in adults and adolescents weighing at least 77 pounds. However, it is not recommended for individuals at risk of acquiring HIV through receptive frontal sex.
Apretude® (Cabotegravir – CAB): A long-acting injectable PrEP that is recommended for all adults and adolescents weighing at least 77 pounds. It is an effective option to reduce the risk of HIV transmission through sexual contact or injection drug use. Apretude® can be beneficial for individuals who struggle with adhering to daily oral PrEP, prefer receiving injections every two months over taking oral medication, or have severe kidney disease preventing the use of oral PrEP drugs.
PrEP Effectiveness
Oral PrEP Effectiveness
When taken as prescribed, oral PrEP can be up to 99% effective at preventing HIV in men who have sex with men (MSM), and heterosexual men and women.1
Oral PrEP is effective up to 84% in people who inject drugs (PWID).1
Oral PrEP reaches maximum protection from HIV for receptive anal sex at about 7 days of daily use.2
For receptive vaginal sex and injection drug use, PrEP reaches maximum protection at about 21 days of daily use.2
Please note, Descovy has not had any trials for vaginal sex. This is an important consideration for cis-women and trans-men.3
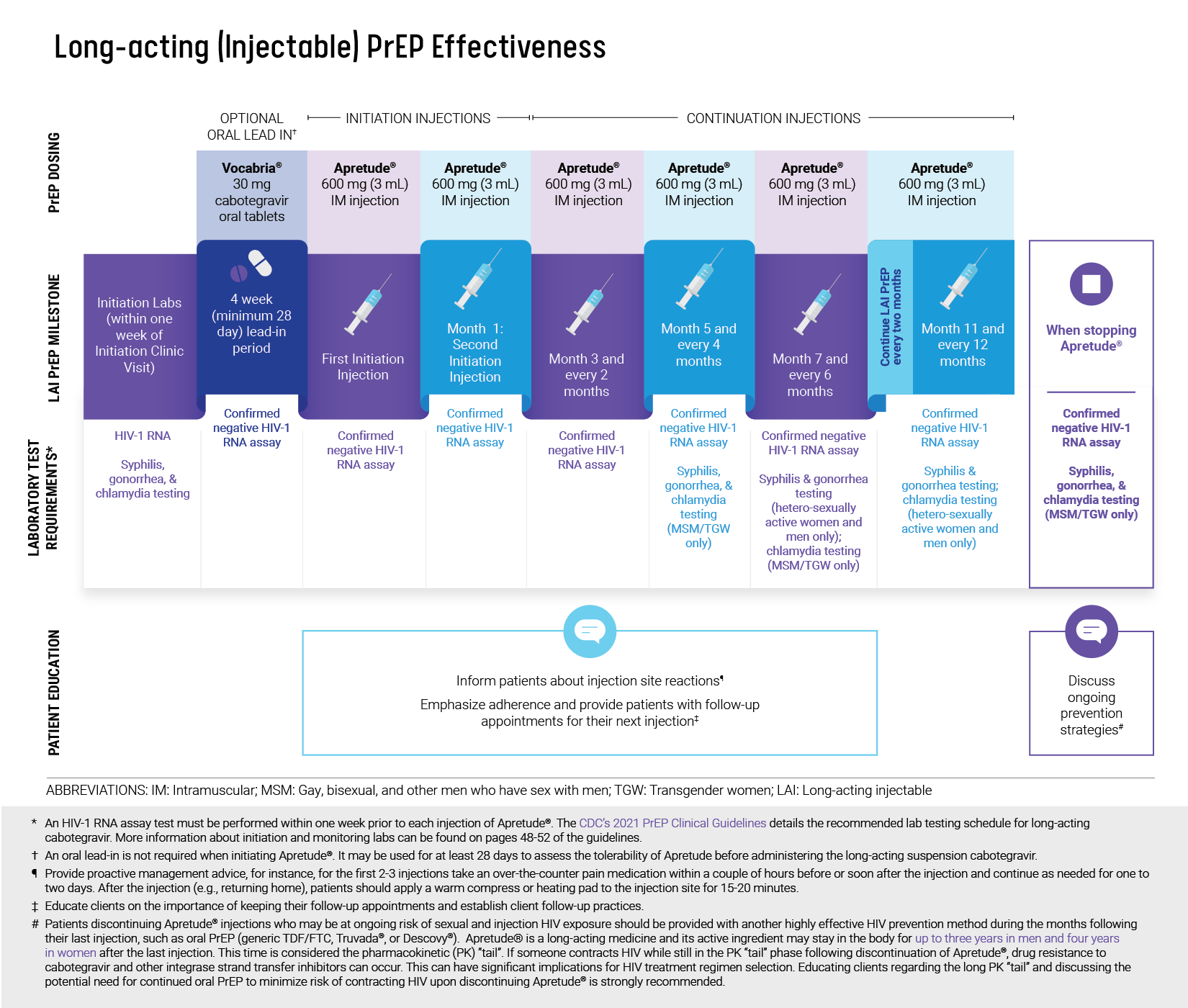
Long-acting (Injectable) PrEP Effectiveness
Adhering to a bimonthly injection schedule is crucial to maintain protective levels of medication.4
Long-acting cabotegravir has shown superiority in HIV prevention when compared to daily oral PrEP in randomized trials.4,5
Currently, there is a lack of available data to estimate the timeframe required for achieving optimal drug levels associated with protection against HIV acquisition when using the injectable PrEP (CAB).
For More Information
PrEP Effectiveness | CDC
cdc.gov/hiv/prevention/prep.html?CDC_AAref_Val=https://www.cdc.gov/hiv/basics/prep/prep-effectiveness.html#cdc_prevention_health-prep-effectivenessEmtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial
pubmed.ncbi.nlm.nih.gov/32711800Antiretroviral prophylaxis for HIV prevention in heterosexual men and women
pubmed.ncbi.nlm.nih.gov/22784037
Image source: “Long-Acting InjectableCabotegravir Dosing” at nastad.org/sites/default/files/2022-06/Microsite-LAI-Infographic-Cabotegravir-Dosing.pdf
Do Not Prescribe Cabotegravir to the following patients:
Individuals allergic to Cabotegravir
Individuals who are taking any of the following medicines:
Carbamazepine
Oxcarbazepine
Phenobarbital
Phenytoin
Rifampin
Rifapentine
On-Demand PrEP
On-Demand PrEP or PrEP 2-1-1 is a non-daily PrEP dosing strategy that has been studied with tenofovir disoproxil fumarate/emtricitabine (Truvada) and has been shown to be effective at preventing HIV transmission in MSM and transgender women.6
PrEP 2-1-1 can prevent HIV transmission during anal sex.6
PrEP medication absorbs slower into vaginal tissue than anal tissue, so PrEP 2-1-1 is not an effective option for vaginal sex.7
PrEP 2-1-1 can be an option for people who have less frequent anal sex or for people who are unable or prefer not to take daily or injectable PrEP.6
On-Demand PrEP Dosing8
Two pills between 2 and 24 hours before sex. Taking the pills closer to 24 hours before sex is better but can be taken up to 2 hours before sex.
After sex, one pill 24 hours after the first pills, and one pill again 24 hours after that.
Note that On-Demand PrEP has not been FDA approved and is not recommended by CDC. Daily PrEP has extensive clinical trial data on safety and efficacy and is the only dosing strategy recommended by the CDC.
For More Information
On-Demand PrEP | CDC
cdc.gov/hiv/basics/prep/on-demand-prep.htmlCDC Clinical Guidelines: Preexposure Prophylaxis for the Prevention of HIV Infection (pg. 55-57)
cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
Baseline and follow-up labwork every 3 months are the same as daily PrEP labwork. See “Prescribing PrEP” section below.
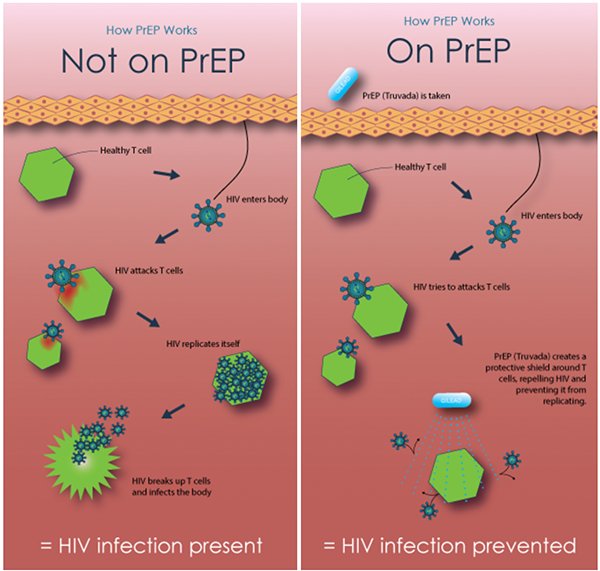
How PrEP Works
Image source: The HIV Prevention Trials Network. Available at hptn.org/resources/education.
PrEP Continuum
The PrEP Continuum mirrors the HIV Continuum of Care, albeit with a focus on HIV-negative individuals. It serves as a tool for monitoring efforts aimed at raising awareness, utilization, and adherence to PrEP among individuals who are at risk of contracting HIV.
The PrEP Continuum consists of four parts:
Awareness of PrEP
Discussing PrEP with a medical provider in the past year
PrEP usage in the past year
PrEP adherence in the past year
Philadelphia Estimates8
There were 266,303 people in Philadelphia are estimated to be at risk for acquiring HIV in 2022.
Among these, 8,750 had an indication for PrEP.
The greatest overall number and proportion of persons with an indication for PrEP was among MSM.
Continuum of PrEP Awareness and Usage in Philadelphia8
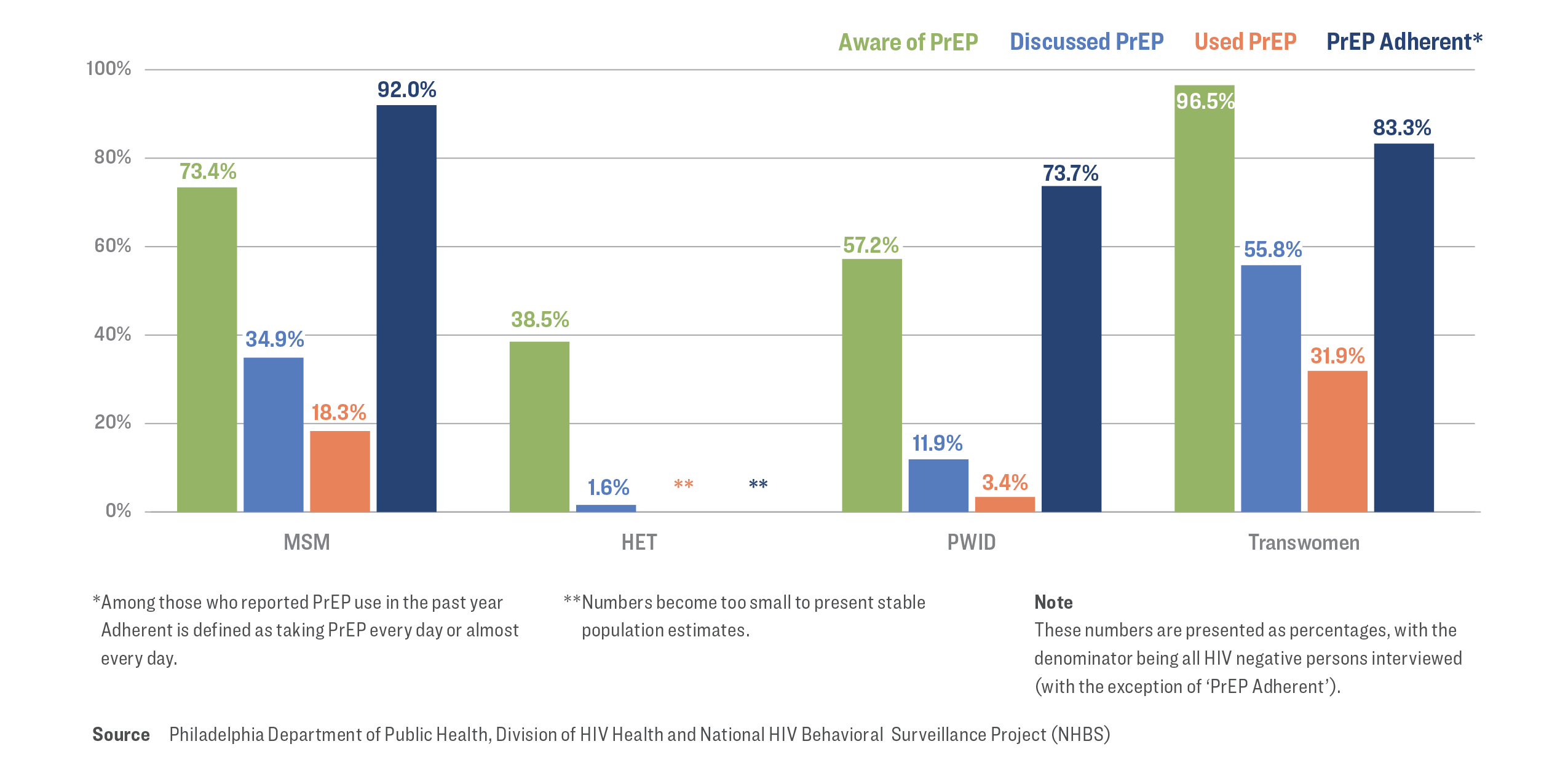
Note: Daily PrEP adherence was self-reported by participants. Possibility of overestimation of true PrEP adherence exists and may impact findings.
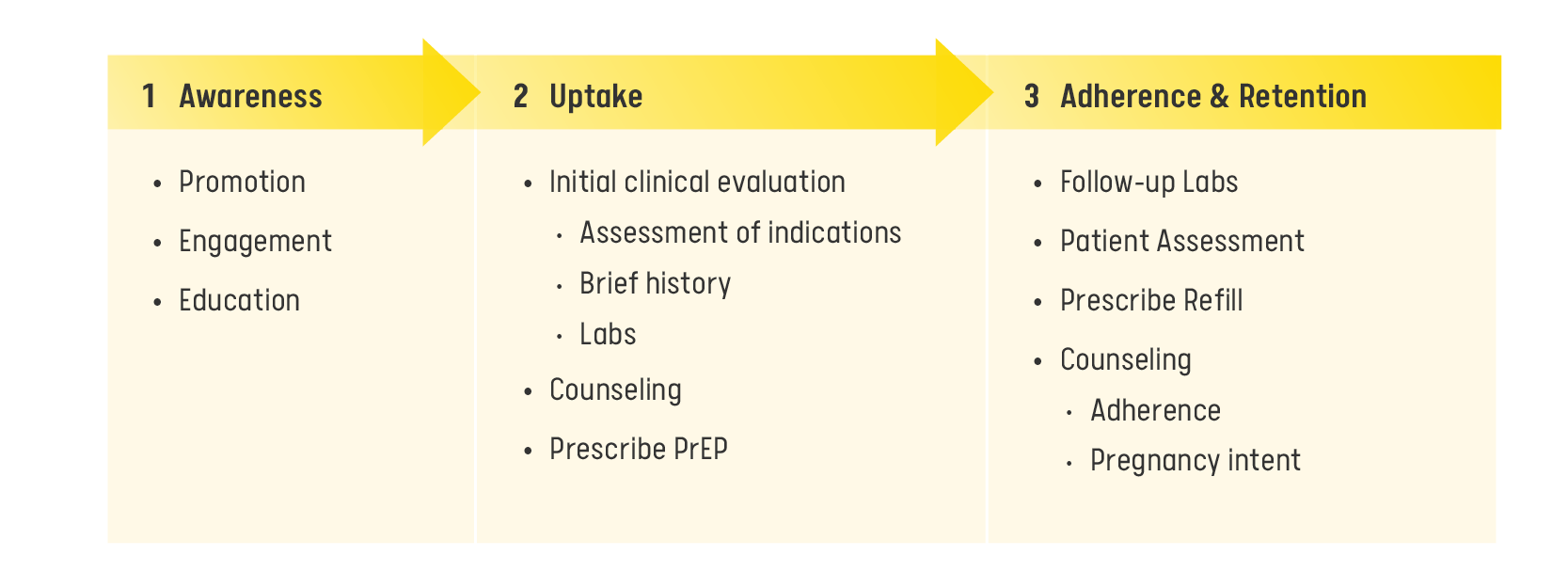
PrEP Clinical Care Model
Within the PrEP clinical care model, the process involves:
Identifying and engaging patients in need of PrEP;
Conducting essential examinations and laboratory assessments;
Prescribing PrEP to eligible patients and subsequently;
Maintaining patient oversight with follow-up appointments and prescription renewals for as long as the patient needs PrEP.
Navigating Conversations About PrEP
A conversation about PrEP and patient’s interest should be started with anyone with a risk of acquiring HIV (particularly patients who are having sex and patients who are injecting drugs) and anyone who requests PrEP for HIV prevention.
A sexual history assessment is essential in discussing HIV risk, PrEP indication, and risk for other STIs, Hepatitis infections, or pregnancy. Please see Getting Started for further detailed guidance and information for sexual health and wellness.
A patient might initially decline PrEP if they do not perceive its relevance at that moment, and providers may hesitate to reintroduce the topic. However, individuals may go through phases where their risk for HIV acquisition and interest in PrEP fluctuates.
Additionally, it is important for providers to address stigma and empower patients in health-promoting behaviors.
PrEP Indications
Indications for PrEP in Sexually Active Persons
In 2022, MSM accounted for 51% of newly diagnosed HIV infections in Philadelphia and heterosexual accounted for 20.7% of newly diagnosed HIV infections.9
The following can increase risk of HIV acquisition in sexually active adults and adolescents at risk of HIV acquisition:
Anal or vaginal sex in the past 6 months and
HIV positive sex partner with unknown or detectable viral load and
History of inconsistent condom use or no condom use with sexual partners and
Identifies as men who has sex with men or transgender woman and tests positive for syphilis, gonorrhea, or chlamydia in the last 6 months; or identifies as heterosexual and tests positive for syphilis or gonorrhea in the last 6 months10
PrEP should be discussed with everyone who is sexually active and prescribed if requested, even if sexual history shows no indications.
Indications for PrEP in People Who Inject Drugs (PWID)
In 2022, PWID accounted for 13.9% of newly diagnosed HIV infections in Philadelphia.9
The following are PrEP indications in adults or adolescents at risk of HIV acquisition through injection use:
Any injection drug use in the past 6 months
Sharing of injection or drug preparation equipment in the past 6 months10
PrEP should be discussed with every person who injects drugs and should be prescribed if requested.
Do Not Withhold PrEP from Candidates Who:
Are pregnant or planning a pregnancy.
Use other risk-reduction practices inconsistently, including condoms.
Report substance use.
Have mental health disorders, including those with serious persistent mental illness.
Report intimate partner violence.
Have unstable housing or limited social support.
Recently were diagnosed with a sexually transmitted infection (STI).
Request PrEP even if they have a partner living with HIV with an undetectable viral load.
Disclose that they are not currently sexually active, as their sexual practices are subject to change over time.
Prescribing PrEP
Starting Patients on PrEP
PrEP should be discussed with all sexually active adults and adolescents. Patients and other clinicians may have concerns that PrEP increases risky behavior, and should not be prescribed to patients to avoid promoting such behaviors. This misconception is stigmatizing and limits the benefit of this biomedical prevention approach that should be used among anyone with any level of risk for HIV acquisition.10
Prescribers must obtain the following labs at baseline prior to initiating PrEP:
Negative HIV Ag/Ab or HIV RNA PCR and
CMP — Kidney function tests, liver function tests and
RPR screening — syphilis and
Chlamydia and gonorrhea screening at all possible sites and
Lipid panel (if starting Descovy) and
Hepatitis B serology and
Hepatitis C antibody screening (with reflex to PCR confirmatory testing) and
Urine pregnancy test for people who can get pregnant.9
Patients should be counseled on the dosing of the prescribed modality, importance of adherence, side effects, time to protection, and follow-up/ testing schedule. Patients should also be educated that PrEP does not prevent other STIs or pregnancy.10
For More Information
- PrEP 2021 CDC Guidelines
cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
What is TelePrEP?
TelePrEP is a program designed to offer convenient online PrEP services and medication delivery to residents of Philadelphia, all within the comfort of their homes.
The array of services encompasses:
Virtual appointments with PrEP providers
At-home lab work
Complimentary delivery of PrEP medications
Transitioning from PEP to PrEP
Individuals who are considered candidates for PrEP after PEP, are:
Individuals who request PrEP and may have experienced an HIV exposure incident due to sexual activity or injection drug use within the preceding 72 hours.
Individuals who have undergone multiple rounds of PEP within a relatively short time frame, such as more than twice in the past 6 months.
Paying for PrEP
Insurance and Medicaid Coverage
Most insurance plans and Medicaid programs include coverage for PrEP.
Under the Affordable Care Act, PrEP is typically available free of charge under nearly all health insurance plans.
This means patients will not incur any expenses for PrEP medication or the necessary clinic visits and lab tests to maintain their prescription.
PrEP-related ICD-10 Codes:
Z20.6 Contact with and (suspected) exposure to HIV
Z20.2 Contact with and (suspected) exposure to infections with a predominantly sexual mode of transmission
Z11.3 Encounter for screening for infections with a predominantly sexual mode of transmission
Z11.4 Encounter for screening for human immunodeficiency virus
Z11.59 Encounter for screening for other viral diseases
Z79.899 Other long term drug therapy
- Z29.81 Encounter for HIV pre-exposure prophylaxis
Access Without Insurance or Medicaid
If an individual has no insurance or Medicaid coverage, there are alternative programs that offer PrEP at no cost or reduced rates:
Co-pay Assistance Programs: these programs reduce the cost of PrEP medications, with eligibility not based on income
ViiVConnect: offers a program to assist with covering the expenses of Apretude injections
Gilead Advancing Access Program: assists in paying for Truvada and Descovy
References
1. Centers for Disease Control and Prevention. (2019). Effectiveness of prevention strategies to reduce the risk of acquiring or transmitting HIV. Centers for Disease Control and Prevention; U.S. Department of Health and Human Services.
cdc.gov/hiv/risk/estimates/preventionstrategies.html#anchor_1562942347
2. Centers for Disease Control and Prevention. (2020). PrEP effectiveness. Centers for Disease Control and Prevention; U.S. Department of Health and Human Services.
cdc.gov/hiv/basics/prep/prep-effectiveness.html
3. U.S. Food and Drug Administration . (2021). Highlights of prescribing information. U.S. Food and Drug Administration.
accessdata.fda.gov/drugsatfda_docs/label/2021/208215s017lbl.pdf
4. Landovitz, R. J., Donnell, D., Clement, M. E., Hanscom, B., Cottle, L., Coelho, L., Cabello, R., Chariyalertsak, S., Dunne, E. F., Frank, I., Gallardo-Cartagena, J. A., Gaur, A. H., Gonzales, P., Tran, H. V., Hinojosa, J. C., Kallas, E. G., Kelley, C. F., Losso, M. H., Madruga, J. V., & Middelkoop, K. (2021). Cabotegravir for HIV Prevention in Cisgender Men and Transgender Women. New England Journal of Medicine, 385(7), 595–608.
doi.org/10.1056/nejmoa2101016
5. Landovitz, R. J., Hanscom, B., Clement, M. E., Tran, H. V., Kallas, E. G., Magnus, M., Sued, O., Sánchez, J., Scott, H., Eron, J., Carlos del Río, Fields, S. D., Marzinke, M. A., Eshleman, S. H., Donnell, D., Spinelli, M. A., Kofron, R., Berman, R., Piwowar-Manning, E., & Richardson, P. A. (2023). Efficacy and safety of long-acting cabotegravir compared with daily oral tenofovir disoproxil fumarate plus emtricitabine to prevent HIV infection in cisgender men and transgender women who have sex with men 1 year after study unblinding: A secondary analysis of the phase 2b and 3 HPTN 083 randomised controlled trial. The Lancet HIV, 10(12), e767–e778.
doi.org/10.1016/s2352-3018(23)00261-8
6. Molina, J.-M., Ghosn, J., Assoumou, L., Delaugerre, C., Algarte-Genin, M., Pialoux, G., Katlama, C., Slama, L., Liegeon, G., Beniguel, L., Ohayon, M., Mouhim, H., Goldwirt, L., Spire, B., Loze, B., Surgers, L., Pavie, J., Lourenco, J., Ben-Mechlia, M., & Le Mestre, S. (2022). Daily and on-demand HIV pre-exposure prophylaxis with emtricitabine and tenofovir disoproxil (ANRS PREVENIR): A prospective observational cohort study. The Lancet HIV, 9(8), e554–e562.
doi.org/10.1016/S2352-3018(22)00133-3
7. Seifert, S. M., Glidden, D. V., Meditz, A. L., Castillo-Mancilla, J. R., Gardner, E. M., Predhomme, J. A., Rower, C., Klein, B., Kerr, B. J., Guida, L. A., Zheng, J.-H., Bushman, L. R., & Anderson, P. L. (2014). Dose response for starting and stopping HIV preexposure prophylaxis for men who have sex with men. Clinical Infectious Diseases, 60(5), 804–810.
doi.org/10.1093/cid/ciu916
8. San Francisco AIDS Foundation. (2024). Resource: Q&A: PrEP 2-1-1. San Francisco AIDS Foundation.
sfaf.org/resourcelibrary/qa-prep-2-1-1-for-anal-sex
9. Philadelphia Department of Public Health Division of HIV Health. (2023). HIV Surveillance Report 2022. City of Philadelphia.
phila.gov/media/20231228103020/HIVSurveillance_Report_2022.pdf
10. Centers for Disease Control and Prevention. (2021). Preexposure prophylaxis for the prevention of HIV infection in the United States – 2021 update, A clinical practice guideline. In Centers for Disease Control and Prevention. U.S. Department of Health and Human Services.
cdc.gov/hiv/pdf/risk/prep/cdc-hiv-prep-guidelines-2021.pdf
Baeten, J. M., Donnell, D., Ndase, P., Mugo, N. R., Campbell, J. D., Wangisi, J., Tappero, J. W., Bukusi, E. A., Cohen, C. R., Katabira, E., Ronald, A., Tumwesigye, E., Were, E., Fife, K. H., Kiarie, J., Farquhar, C., John-Stewart, G., Kakia, A., Odoyo, J., & Mucunguzi, A. (2012). Antiretroviral prophylaxis for HIV prevention in heterosexual men and women. New England Journal of Medicine, 367(5), 399–410.
doi.org/10.1056/nejmoa1108524
Delany-Moretlwe, S., Hughes, J. P., Bock, P., Ouma, S. G., Hunidzarira, P., Kalonji, D., Kayange, N., Makhema, J., Mandima, P., Mathew, C., Spooner, E., Mpendo, J., Mukwekwerere, P., Mgodi, N., Ntege, P. N., Nair, G., Nakabiito, C., Nuwagaba-Biribonwoha, H., Panchia, R., & Singh, N. (2022). Cabotegravir for the prevention of HIV-1 in women: results from HPTN 084, a phase 3, randomised clinical trial. The Lancet, 399(10337), 1779–1789.
doi.org/10.1016/S0140-6736(22)00538-4
Mayer, K. H., Molina, J.-M., Thompson, M. A., Anderson, P. L., Mounzer, K. C., De Wet, J. J., DeJesus, E., Jessen, H., Grant, R. M., Ruane, P. J., Wong, P., Ebrahimi, R., Zhong, L., Mathias, A., Callebaut, C., Collins, S. E., Das, M., McCallister, S., Brainard, D. M., & Brinson, C. (2020). Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): Primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. The Lancet, 396(10246), 239–254.
doi.org/10.1016/s0140-6736(20)31065-5